Cesin Cold Chain Validation Services
Under the regulations of GSP (Good Supply Practice) andGMP (Good Manufacturing Practice), validation typically refers to the confirmation of critical elements, processes, or activities within the quality management system of pharmaceutical trading enterprises, such as facilities and equipment, management systems, personnel qualifications, documentation and records, as well as production processes.This process ensures that they comply with predetermined standards and requirements, thereby safeguarding the quality and safety of pharmaceutical products.
With ISO and CNAS certified validation laboratory, professional technical team and advanced validation equipments,Guangzhou Cesin Cold Chain is committed to pro.vide third-party professional cold chain validation services for pharmaceutical and medical fields in compliance with national regulations.
With ISO and CNAS certified validation laboratory, professional technical team and advanced validation equipments,Guangzhou Cesin Cold Chain is committed to pro.vide third-party professional cold chain validation services for pharmaceutical and medical fields in compliance with national regulations.

On-site
verification
verification
Remote
verification
verification
Verification equipment
leasing.
leasing.
We meticulously craft three major validation business segments: efficient on-site validation for saving time and efort;remote validation services to reduce costs and enhance efficiency; and validation equipment rental for flexibility and cost-effectiveness.All-round to meet your needs.

We specialize in offering a comprehensive suite of validation services for all types of facilities and equipment utilized in thestorage and transportation of refrigerated and frozen pharmaceuticals, including cold storages, refrigerated vehicles, coolerboxes, freezers, as well as temperature and humidity monitoring systems. This allows us to fully cater to the prevalent de-mands for cold chain validation in the market.in addition, all of our cold chain packaging series conform to global industrystandards such as the International Safe Transit Association (lSTA) and the World Health Organization (WHO).
We have independently developed an innovative
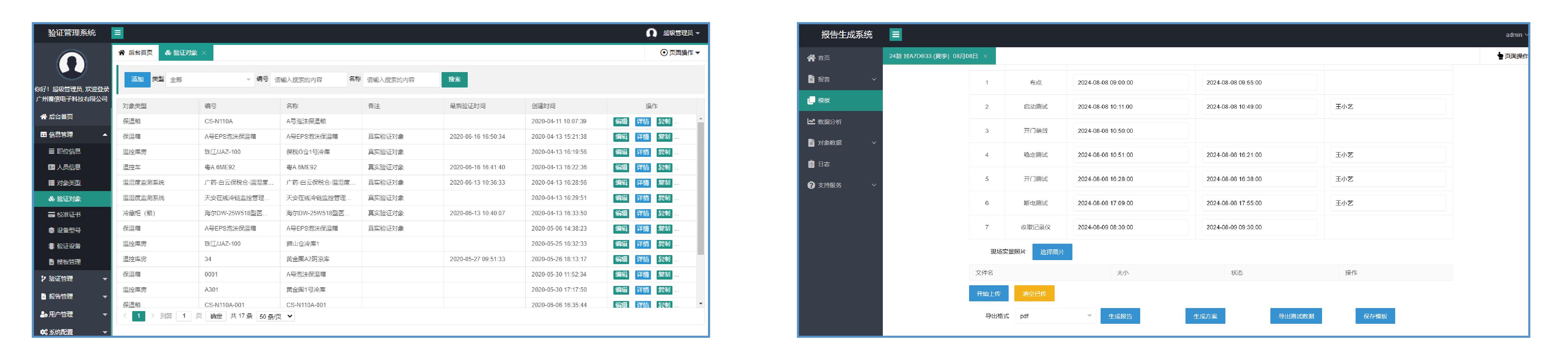
LoRa wireless loT recorder and an efficient GSP intelligent validation platform, which are designed to.ensure ultra-stable transmission of validation data and enhance the efficiency of the validation processReal-time monitoring of the whole process and ac.curate presentation of test results. The whole validation process is visualized, transparent and efficient.
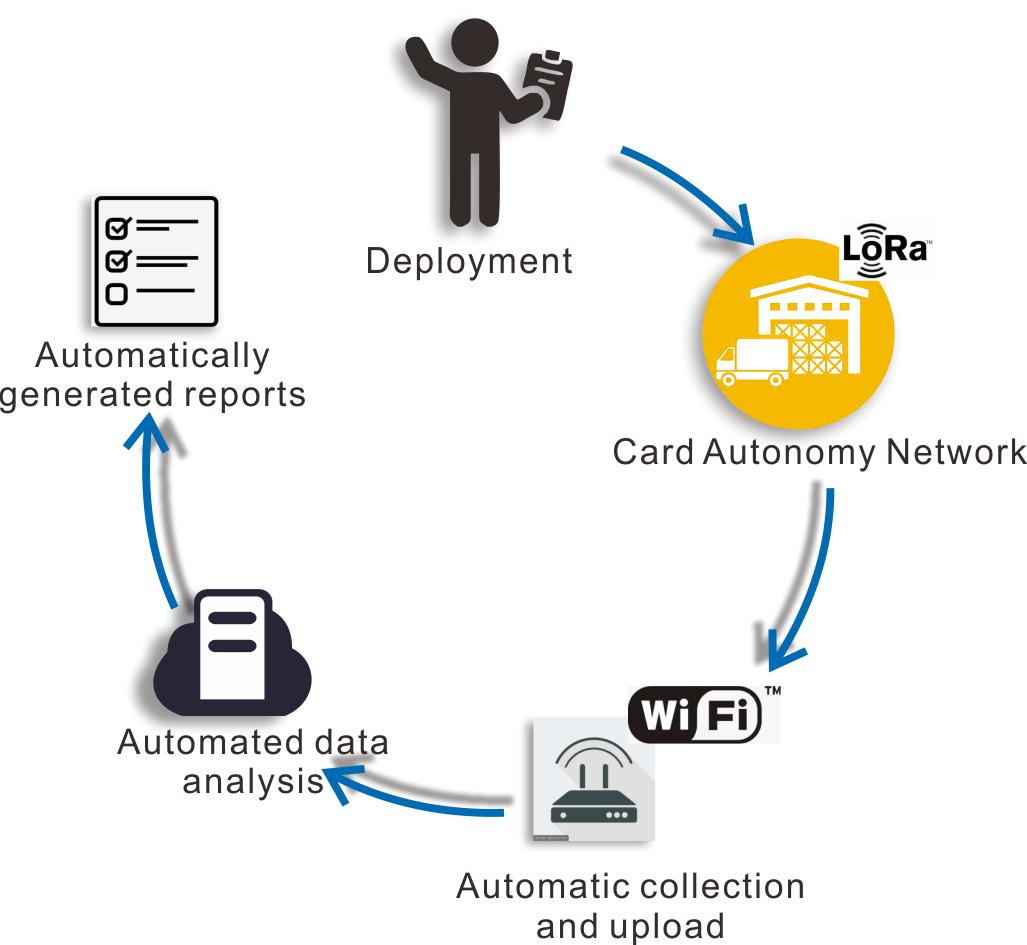

One-stop solution, save time and effort
• Advanced equipment, precision and accuracy
• Professional team. safety and reliability
• Efficient and time-saving, flexible and cost-effective
• Well-established system, efficient and orderly
• Customized services, precisely tailored to your needs
• Advanced equipment, precision and accuracy
• Professional team. safety and reliability
• Efficient and time-saving, flexible and cost-effective
• Well-established system, efficient and orderly
• Customized services, precisely tailored to your needs
Contact us to find the optimal cold chain transportation rental program for your business needs!

